How to Find the Number of Atoms in Aluminum Foil
Next you must convert the mass of the sample to moles by using the molar mass of aluminium which is equal to 26982 g mol1. We took squares of aluminum foil measured the surface area and mass.

This Is The Part Of A Series On The Atom It Covers The Key Experiments That Changed The Model Of The Ato Chemistry Classroom Electron Configuration Bohr Model
1 mole of aluminium has a mass of 27 g.
. This can be accomplished using the formula. An entire roll of aluminum foil is 375 ft2. Multiply the number of moles of aluminum by Avogadros Number 602x1023 atomsmole.
Calculate how many atoms of aluminum there are in the thickness of the foil now that you know the diameter of a single atom and the thickness of the foil in cm. To determine the number of atoms thick the foil was dimensional analysis was used to convert the centimeter thickness to number of atoms. Divide the grams of aluminum by the molar mass of aluminum 2698gmole to convert mass to moles.
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Find the height thickness of the foil in cm from the relation Volume l w h and record the result.
Use dimensional analysis to find the thickness of the foil in atoms. Chemistry need help. An entire roll of aluminum foil is 375 ft2.
The hypothesis states that if an aluminum sheet is thicker then it will have more atoms. Cut out three squares of aluminum foil with sides of the following lengths. 02003moles Al Avogadros constant 6022 1023atoms Al 1mole Al 121 1023atoms Al The answer is rounded to three sig figs the number of sig figs you have for the volume of the foil.
To determine the number of atoms in the whole piece of foil the volume of the foil was converted to atoms using dimensional analysis. Approximately 193000 atoms thick. Hence the mass of foil is 386 grams.
Atoms in your foil_____ Calculation of atoms per person. Record the sizes in the data table 4. What is the number of atoms in aluminum.
This is a fairly rough estimate from classroom data. Then multiply by avagradros number. The value was 286 10-3 cm The.
Let the base area be 1. Up to 24 cash back One aluminum atom is 25 x 10-8 cm thick. Number of particles has a mass of 27 grams.
How do you find the diameter of an aluminum atom. You will need to use scientific notation Is it more than 10000 atoms thick. By dividing the mass by a known density value we obtained the volume of aluminum foil in our sample.
Safety cautions in this experiment include no horseplay with the rulers. To determine the number of atoms in the whole piece of foil the volume of the foil was converted to atoms using dimensional analysis. Solve for H height which is the thickness.
To determine the number of atoms thick the foil was dimensional analysis was used to convert the centimeter thickness to number of atoms. How many protons do aluminum. Finally to convert the number of moles to atoms use the fact that 1 mole of aluminium must contain 60221023 atoms of aluminium this is known as Avogadros constant.
So number of particles has a mass of. One aluminum atom is 25 x 10-8 cm thick. How do you find the number of atoms in aluminum foil.
Next you must convert the mass of the sample to moles by using the molar mass of aluminium which is equal to 26982 g mol. An entire roll of aluminum foil is 375 ft2. Divide the thickness of the foil by the diameter of an atom to find out how many atoms thick the foil is.
Measure the mass of each of the squares of foil and record in the data table. Population of the world _____ round to 2 or 3 digits 6. V L W H.
The purpose of this experiment is to find the number of atoms that forms thickness of the aluminum foil and to find the thickness of a piece the aluminum foil in centimeters by finding the mass volume area length and width of the foil using the density of the aluminum which is 270gcm cubed and the radius of the atom 143 x 10 to the -8. Using the mass of the aluminum foil compute the number of moles of aluminum and the total number of atoms of aluminum in your. An entire roll of aluminum foil is 375 ft 2.
To determine the number of atoms in the whole piece of foil the volume of the foil was converted to atoms using dimensional analysis. The null hypothesis states that if an aluminum sheet is thinner then it will have less atoms. Number of particles are contained in 1 mole of an element.
To determine the number of atoms in the whole piece of foil the volume of the foil was converted to atoms using dimensional analysis. Number of aluminium atoms To calculate the mass of given number of atoms we use unitary method. Data Foil Piece Length cm Width cm Area cm2 Mass g Density gcm3 Volume cm3 Thickness cm Heavy 100 102 102 066 26989 245 240x10-3 Thin 104 910 9464 044 26989 163 172x10-3 Three ways to calculate VmD.
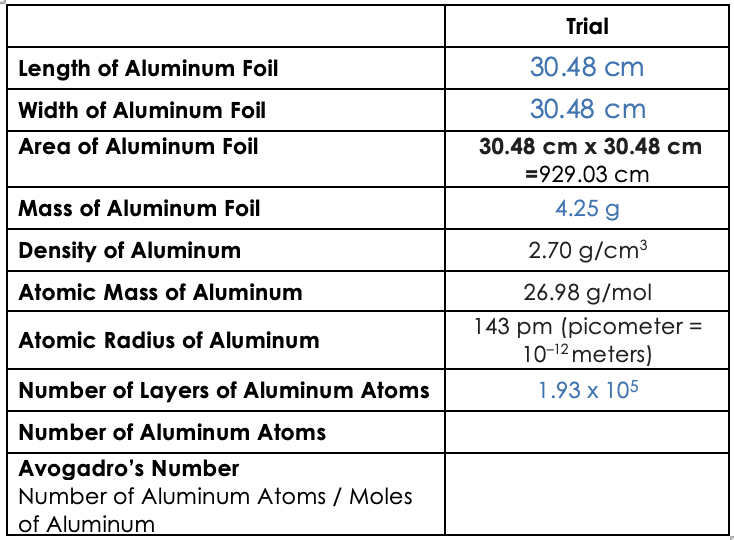
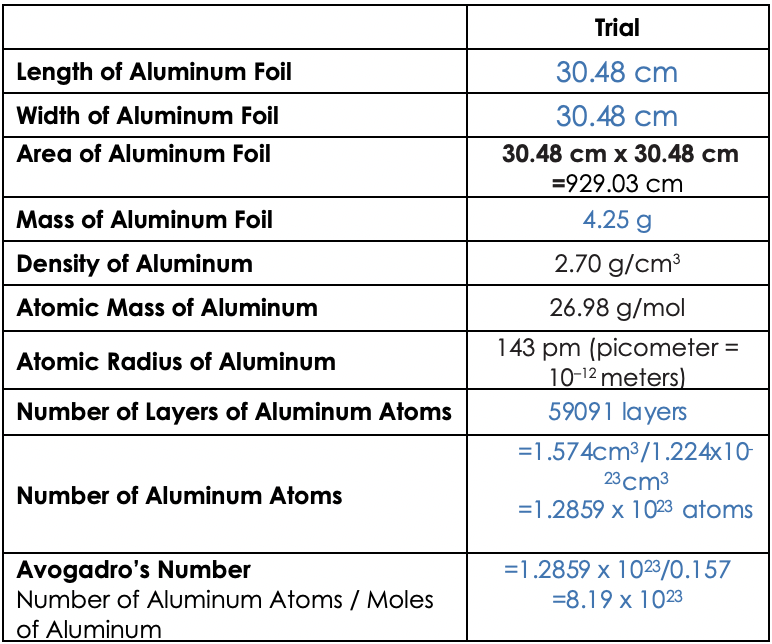
Up to 24 cash back 4. Thickness_____ diameter of an atom 286 10-8 cm. Trial Length of Aluminum Foil 3048 cm Width of Aluminum Foil Area of Aluminum Foil 3048 cm 3048 cm x 3048 cm 92903 cm Mass of Aluminum Foil 425 g Density of Aluminum Atomic Mass of Aluminum Atomic Radius of Aluminum 270 gcm 2698 gmol 143 pm picometer 10-12 meters 59091 layers 1574cm31224x10 23 cm3 12859 x 1023.
Materials and Procedure Heavy Aluminum Foil Thin Aluminum Foil Ruler Balance. How many atoms of aluminum are there in 125 g of aluminum foil. 40 cm 70 cm and 100 cm.
Use Avogadros number to determine the number of atoms in the piece of matter. Determine the area of the squares and record them in the data table. _____ Calculation of atoms thick.
And the number of atoms in the thickness of foil. Number of height J x 1 atom___ Atoms thick 25 x 10-8cm Calculate the number of moles of aluminum and the total number of atoms of. By dividing that by the surface area we found the thickness of the aluminum foil in cm.
Find the thickness of the foil in atoms. To determine the number of atoms thick the foil was dimensional analysis was used to convert the centimeter thickness to number of atoms. By dividing that by the surface area we found the thickness of the aluminum foil in cm.
If there are 602214076x1023 anythingin this case atomsin a mole and your asking how many atoms of Al there are in 362 moles of AluminumAl then the answer is602214076x1023x362218001495512x1024 there are 218001495512x1024 atoms of Al in 362 moles of AluminumAl atoms. By dividing the mass by a known density value we obtained the volume of aluminum foil in our sample.

Ep1940526b1 Multi Track Multi Vehicle Roller Coaster Patent Drawing Patent Art Technical Drawing
Solved Write Out A Step By Step Procedure For Determining Chegg Com

Mole To Mass Calculations Work Energy And Power Energy Work Chemistry Notes

Easy And Engaging Ways To Teach Force And Motion In Kindergarten Mrs B S Beehive Kindergarten Skills Force And Motion Kindergarten Resources

Neodymium Ree Handbook Astronomy Science Rocks And Minerals Atomic Structure

Transparent Translucent And Opaque Game And Posters Light Science Fun Science Science Games
Solved Determine The Number Of Layers Of Atoms Assuming Chegg Com

Paper Drop Design Competition Activity Design Competitions Engineering Design Process Drops Design

Aluminum Uses Properties Compounds Britannica

Balancing Chemical Equations Worksheet Chemical Equation Chemistry Worksheets Balancing Equations
How To Find The Thickness Of Aluminum Foil In Atoms Quora

Sulfur Atom School Project With Candy In A Drawer School Projects Beaded Projects Beaded School Projects Projects

When You Dissolve Copper Chloride Into Water It Makes A Solution When You Put Pure Aluminum Foil Into The Solution A Science Skills Chemical Energy Desserts

Nuclear 101 Youtube Nuclear Energy Nuclear Energy Work

Mixtures And Solutions Worksheet Answers Elegant Homogeneous Or Heterogeneous Mixt Compounds And Mixtures Heterogeneous Mixture Elements Compounds And Mixtures



Comments
Post a Comment